Interesting in testing your medical device?
Contact us and we will support you with samples and to carry on the test.
Contact us

We notice that you are visiting us from . This site only services Europe, the Middle East, and Asia-based visitors. Would you like to visit the site that is appropriate for your location?
Compatibility is the assurance that the device or surface and the cleaning and disinfectant solutions are suitable for use together. Our Compatible by Design™ product range has been designed with compatibility in mind, setting it apart in the field of infection prevention. With PDI, you'll never have to compromise clinical efficacy to safeguard your equipment.
Find out more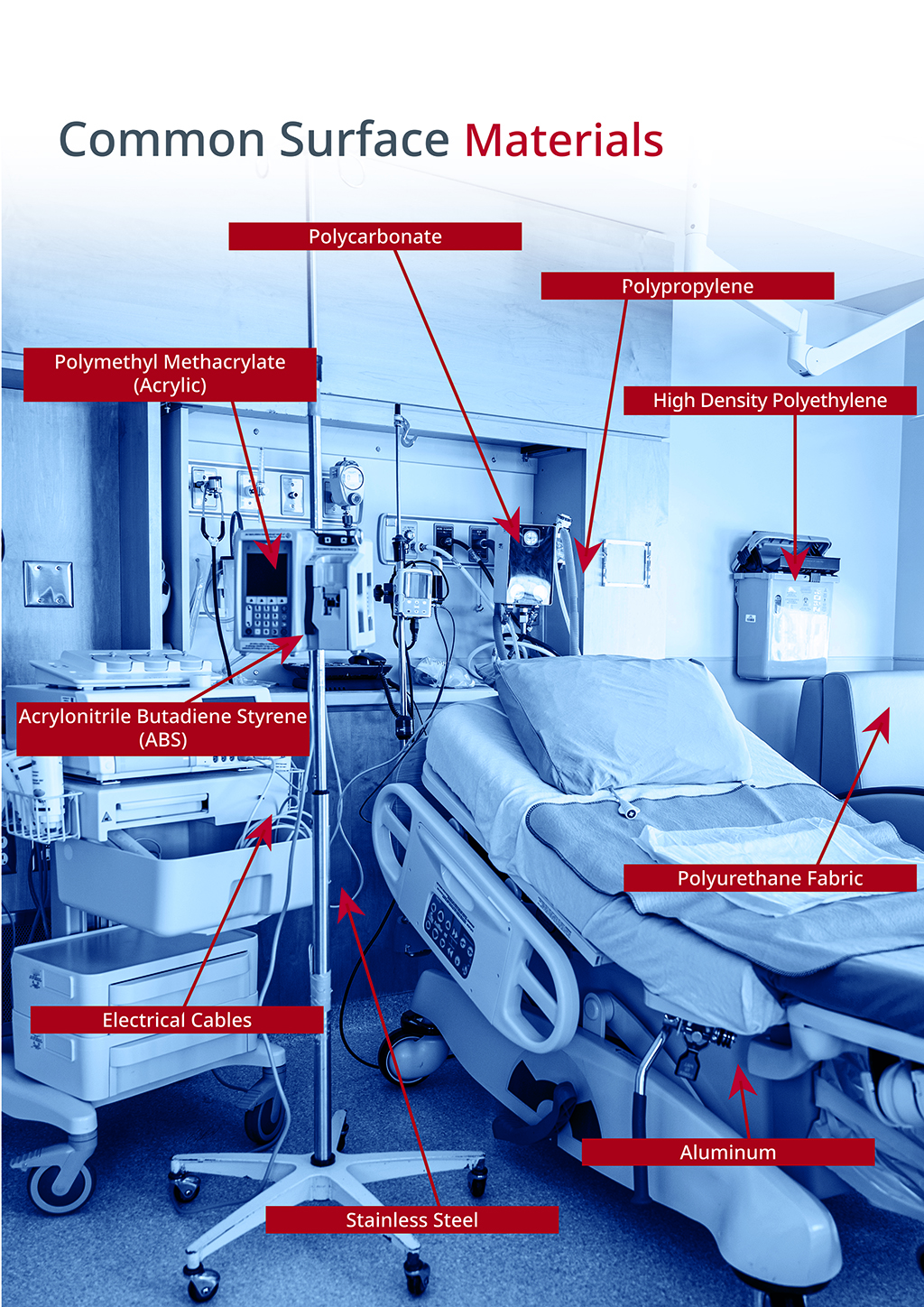

Ensuring proper compatibility between disinfectants and medical devices is a critical aspect of infection prevention in healthcare settings. There are a number of steps healthcare facilities can take to improve overall adherence to the correct disinfecting protocols while maintaining medical device integrity:
1: Understand the decontamination level required
2: Refer to the Medical Equipment IFU (Instructions for Use)
3: Seek compatibility data from solution providers
4:Carry out thorough training
Find out more

Have you checked whether your medical device enclosures can handle harsh disinfectants? Are you sure you’re using materials that won’t degrade with frequent cleaning?
If you need to test how disinfectants affect your devices to include this info in your Instructions for Use (IFUs), we’re here to help.
Find out morePDI is constantly working with equipment manufacturers to test our products on their medical devices to ensure compatibility.
The Sani-Cloth® range has been approved on over 2,000 medical devices and the most common materials used in healthcare facilities.
Download our brochure